Difference between revisions of "Step 3"
(→Step 3 description) |
|||
| (16 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
= Step 3 description = | = Step 3 description = | ||
| − | The purpose of this configuration is to perform a simulation similar to Step 2 but involving now a '''mixture of different gaseous species''' at different temperatures, still '''without any chemical reaction'''. | + | The purpose of this configuration is to perform a simulation similar to '''Step 2''' but involving now a '''mixture of different gaseous species''' at different temperatures, still '''without any chemical reaction'''. |
In this manner, it is possible to assess the accuracy of the numerical models involved in the description of species and heat diffusion | In this manner, it is possible to assess the accuracy of the numerical models involved in the description of species and heat diffusion | ||
| − | The thermodynamic and transport properties of the 9 species kinetic scheme of Boivin et al.<ref name="Boivin2011" | + | The thermodynamic and transport properties of the 9 species kinetic scheme of Boivin et al.<ref name="Boivin2011"/> should be used in preparation for the next step, even though the reactions are still neglected here. |
It should be noted that in this case, the '''density is variable''' in both '''space''' and '''time''' due to the changing composition, which was not the case in the previous step: this induces additional numerical difficulties that must be taken into account. | It should be noted that in this case, the '''density is variable''' in both '''space''' and '''time''' due to the changing composition, which was not the case in the previous step: this induces additional numerical difficulties that must be taken into account. | ||
| Line 19: | Line 19: | ||
To avoid any numerical instability, '''the steps at the interface between both regions are smoothed out using hyperbolic tangent functions''' as follows: | To avoid any numerical instability, '''the steps at the interface between both regions are smoothed out using hyperbolic tangent functions''' as follows: | ||
| − | <math>R_d(x) = |x-0.5\,L|,</math> | + | <math>R_d(x) = |x-0.5\,L|,</math> (1) |
| − | <math>\psi(x) = 0.5\left[1+\mathrm{tanh}\left(\frac{c\,(R_d(x)-R)}{R}\right)\right],</math> | + | <math>\psi(x) = 0.5\left[1+\mathrm{tanh}\left(\frac{c\,(R_d(x)-R)}{R}\right)\right],</math> (2) |
| − | <math>Y_\mathrm{H_2}(x) = Y^0_{\mathrm{H_2}}\left(1-\psi(x)\right),</math> | + | <math>Y_\mathrm{H_2}(x) = Y^0_{\mathrm{H_2}}\left(1-\psi(x)\right),</math> (3) |
| − | <math>Y_\mathrm{O_2}(x) = Y^0_{\mathrm{O_2}}\left(\psi(x)\right).</math> | + | <math>Y_\mathrm{O_2}(x) = Y^0_{\mathrm{O_2}}\left(\psi(x)\right).</math> (4) |
| − | where <math>R = \pi/4 | + | where <math>R = \pi/4</math> mm and <math>c = 3</math> are the half-width of the central slab and stiffness parameter, respectively. |
As a consequence, there is initially a small region where both fuel and oxidizer coexist. | As a consequence, there is initially a small region where both fuel and oxidizer coexist. | ||
Finally, a nitrogen complement is added everywhere using <math>Y_\mathrm{N_2}=1-Y_\mathrm{H_2}-Y_\mathrm{O_2}</math>. | Finally, a nitrogen complement is added everywhere using <math>Y_\mathrm{N_2}=1-Y_\mathrm{H_2}-Y_\mathrm{O_2}</math>. | ||
| Line 35: | Line 35: | ||
* Compute in each cell the equilibrium temperature for the local mixture for constant pressure and enthalpy by using a dedicated solver. | * Compute in each cell the equilibrium temperature for the local mixture for constant pressure and enthalpy by using a dedicated solver. | ||
| − | * Enforce the resulting temperature profile, but keep the species profiles to their initial values (Eqs. | + | * Enforce the resulting temperature profile, but keep the species profiles to their initial values (Eqs. (3) and (4)). |
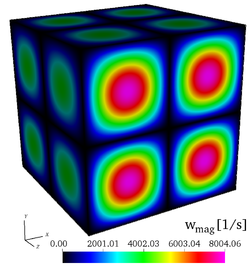
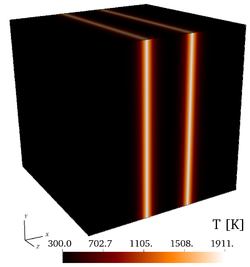
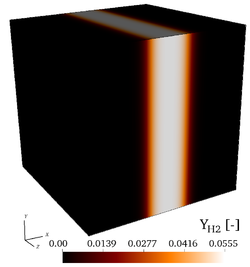
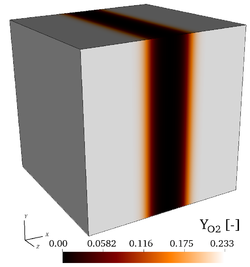
| − | It is found by using '''Cantera''' that the peak adiabatic temperature obtained '''at the fuel/air interface''' is <math>T_{ad}=1910.7~K</math>, leading to the profiles shown in the initial fields of [https://benchmark.coria-cfd.fr/ | + | It is found by using '''Cantera''' that the peak adiabatic temperature obtained '''at the fuel/air interface''' is <math>T_{ad}=1910.7~K</math>, leading to the profiles shown in the initial fields of [https://benchmark.coria-cfd.fr/images/4/40/Wmag_t0.png vorticity], [https://benchmark.coria-cfd.fr/images/6/6d/T_t0.png temperature], |
| + | [https://benchmark.coria-cfd.fr/images/f/fa/H2_t0.png mass fraction of <math>H_2</math>], [https://benchmark.coria-cfd.fr/images/e/ef/O2_t0.png mass fraction of <math>O_2</math>] and the [https://benchmark.coria-cfd.fr/images/b/b4/Profiles_3d_zoom.pdf initial profiles of temperature and of mass fractions]. Users are encouraged to implement the exact initial profiles from the benchmark website to facilitate later comparisons. | ||
The resulting, initial configuration thus involves '''multiple species at different temperatures''' with steep profiles, just like in a real flame. | The resulting, initial configuration thus involves '''multiple species at different temperatures''' with steep profiles, just like in a real flame. | ||
However, for the present simulation, the reaction source terms are all still set to zero, as already mentioned: only '''convection''' and '''diffusion processes''' are considered. | However, for the present simulation, the reaction source terms are all still set to zero, as already mentioned: only '''convection''' and '''diffusion processes''' are considered. | ||
In order to enable benchmark computations also with '''DNS codes''' that do not provide advanced diffusion models, only constant values of the Lewis numbers are considered; for the same reason, thermodiffusion (Soret effect) is neglected on purpose (though it would be obviously relevant for such cases involving hydrogen as a fuel). | In order to enable benchmark computations also with '''DNS codes''' that do not provide advanced diffusion models, only constant values of the Lewis numbers are considered; for the same reason, thermodiffusion (Soret effect) is neglected on purpose (though it would be obviously relevant for such cases involving hydrogen as a fuel). | ||
| − | The authors are fully aware that this is a crude approximation of reality<ref name="pecs" | + | The authors are fully aware that this is a crude approximation of reality<ref name="pecs"/>. But it must be kept in mind that the focus is set here on the detailed comparison between different codes and algorithms, and not on the resulting flow or flame structure. |
| − | Hence, in this and in the next section, the values of the '''Lewis number''' for each species given in | + | Hence, in this and in the next section, the values of the '''Lewis number''' for each species given in the table below shall be used to enable direct comparisons. |
These values have been estimated from a separate DNS for a similar configuration using the mixture-averaged diffusion model. | These values have been estimated from a separate DNS for a similar configuration using the mixture-averaged diffusion model. | ||
The precise description of the associated thermodynamic state is available on the website. | The precise description of the associated thermodynamic state is available on the website. | ||
| Line 75: | Line 76: | ||
On the other hand, considering that implementing advanced models for fluid viscosity and thermal conductivity is not difficult and does not increase the computational time significantly, local values of these quantities depending on composition and temperature should be taken into account; | On the other hand, considering that implementing advanced models for fluid viscosity and thermal conductivity is not difficult and does not increase the computational time significantly, local values of these quantities depending on composition and temperature should be taken into account; | ||
More specifically, and to ease further comparisons, the same models for viscosity and conductivity have been retained in the three participating codes, i.e. the mixture averaged formalism that is used in both the '''Cantera''' and '''Chemkin packages'''. | More specifically, and to ease further comparisons, the same models for viscosity and conductivity have been retained in the three participating codes, i.e. the mixture averaged formalism that is used in both the '''Cantera''' and '''Chemkin packages'''. | ||
| − | |||
The viscosity is variable in the computational domain as well as with time and the '''Reynolds number''' of this configuration can only be estimated using the minimal value of viscosity (which is obtained in the air at 300 K, <math>\nu_\mathrm{min} \approx 1.56 \, 10^{-5} \mathrm{m^2/s}</math>), leading to <math>Re = {u_0 L_0}/{\nu_{min}}=267</math> which guarantees a laminar flow. | The viscosity is variable in the computational domain as well as with time and the '''Reynolds number''' of this configuration can only be estimated using the minimal value of viscosity (which is obtained in the air at 300 K, <math>\nu_\mathrm{min} \approx 1.56 \, 10^{-5} \mathrm{m^2/s}</math>), leading to <math>Re = {u_0 L_0}/{\nu_{min}}=267</math> which guarantees a laminar flow. | ||
| Line 84: | Line 84: | ||
The '''reference time scale''' is here <math>\tau_\mathrm{ref}=L_0/u_0 = 0.25 \; \mathrm{ms}</math>. | The '''reference time scale''' is here <math>\tau_\mathrm{ref}=L_0/u_0 = 0.25 \; \mathrm{ms}</math>. | ||
The simulation should be performed for a physical time of at least <math>t=0.5 \; \mathrm{ms} = 2 \tau_\mathrm{ref}</math> as most of the results presented in this article are taken from this instant. | The simulation should be performed for a physical time of at least <math>t=0.5 \; \mathrm{ms} = 2 \tau_\mathrm{ref}</math> as most of the results presented in this article are taken from this instant. | ||
| − | |||
[[File:wmag_t0.png|250px|alt text]] | [[File:wmag_t0.png|250px|alt text]] | ||
| Line 91: | Line 90: | ||
[[File:O2_t0.png|250px|alt text]] | [[File:O2_t0.png|250px|alt text]] | ||
| − | Initial fields of vorticity magnitude, temperature, mass fractions of <math>\mathrm{H_2}</math>, and mass fraction of <math>\mathrm{O_2}</math> (from left to right), for the 3-D, non-reacting case (Step 3). | + | Initial fields of vorticity magnitude, temperature, mass fractions of <math>\mathrm{H_2}</math>, and mass fraction of <math>\mathrm{O_2}</math> (from left to right), for the 3-D, non-reacting case ('''Step 3'''). |
[[File:profiles_3d_zoom.pdf|250px|alt text]] | [[File:profiles_3d_zoom.pdf|250px|alt text]] | ||
| − | Initial profiles of temperature and of mass fractions at <math>y=0.5 L</math> and <math>z=0.5 L</math> for the 3-D, non-reacting case (Step 3). | + | Initial profiles of temperature and of mass fractions at <math>y=0.5 L</math> and <math>z=0.5 L</math> for the 3-D, non-reacting case ('''Step 3'''). |
= Kinetics = | = Kinetics = | ||
| − | The | + | The kinetic scheme of [https://doi.org/10.1016/j.proci.2010.05.002 Boivin et al.]<ref name="Boivin2011"/> which contains 9 species and 12 reactions has been used for this Benchmark. |
| − | + | ||
| − | + | ||
| + | This mechanism is provided here in the Cantera format: | ||
| + | * [[File:H2_williams_12.xml.zip | ctml ]] | ||
| + | * [[File:H2_williams_12.cti | cti]] | ||
| + | If you are using Chemkin, please use the following files: | ||
| + | * [[File:H2_Boivin_transport.txt | transport]] | ||
| + | * [[File:H2_Boivin_therm.txt | thermodynamic]] | ||
| + | * [[File:H2_Boivin_mech.txt | mechanism]] | ||
= Transport and thermodynamic properties used for this Step = | = Transport and thermodynamic properties used for this Step = | ||
| Line 119: | Line 123: | ||
= References = | = References = | ||
| − | + | <references> | |
| + | <ref name="Boivin2011"> | ||
| + | <bibtex> | ||
| + | @article{Boivin2011, | ||
| + | author= {P. Boivin, C. Jiménez, A.L. Sanchez, and F.A. Williams}, | ||
| + | title= {An explicit reduced mechanism for <math>H_2</math>-air combustion.}, | ||
| + | journal={Proc. Combust. Inst.}, | ||
| + | year= {2011}, | ||
| + | volume={33}, | ||
| + | pages={517--523}, | ||
| + | doi= {https://doi.org/10.1016/j.proci.2010.05.002} | ||
| + | } | ||
| + | </bibtex> | ||
| + | </ref> | ||
| + | <ref name="pecs"> | ||
| + | <bibtex> | ||
| + | @article{Pecs, | ||
| + | author= {R. Hilbert, F. Tap, H. El-Rabii, and D. Thévenin}, | ||
| + | title= {Impact of detailed chemistry and transport models on turbulent combustion simulations}, | ||
| + | journal={Prog. Energ. Combust. Sci.}, | ||
| + | year= {2004}, | ||
| + | volume={30}, | ||
| + | pages={61--117}, | ||
| + | } | ||
| + | </bibtex> | ||
| + | </ref> | ||
| + | </references> | ||
Latest revision as of 15:22, 5 February 2024
Contents
Step 3 description
The purpose of this configuration is to perform a simulation similar to Step 2 but involving now a mixture of different gaseous species at different temperatures, still without any chemical reaction. In this manner, it is possible to assess the accuracy of the numerical models involved in the description of species and heat diffusion The thermodynamic and transport properties of the 9 species kinetic scheme of Boivin et al.[1] should be used in preparation for the next step, even though the reactions are still neglected here.
It should be noted that in this case, the density is variable in both space and time due to the changing composition, which was not the case in the previous step: this induces additional numerical difficulties that must be taken into account. However, there is no variation of the thermodynamic pressure in time, contrary to the next step.
The simulation domain is a cubic box of size with in each direction, where . Compared to the previous step, this smaller size is needed to ensure a proper resolution of the reaction fronts for hydrogen oxidation that will appear in the next step and are associated to fixed characteristic dimensions. Again, periodic boundary conditions are used in all three spatial directions.
The initial velocity field prescribed at is identical to the one used in Step 2 with the reference velocity set to .
In preparation for the next step, the central part of the box is initially filled with a mixture (fuel region, molar fraction , ) while the remaining domain is filled with air (oxidizer region, molar fraction , ). The corresponding mass fractions are and .
To avoid any numerical instability, the steps at the interface between both regions are smoothed out using hyperbolic tangent functions as follows:
(1)
(2)
(3)
(4)
where mm and are the half-width of the central slab and stiffness parameter, respectively. As a consequence, there is initially a small region where both fuel and oxidizer coexist. Finally, a nitrogen complement is added everywhere using .
To also test the behaviour of the codes with respect to heat diffusion, a non-homogeneous temperature profile is finally imposed as follows:
- Compute in each cell the equilibrium temperature for the local mixture for constant pressure and enthalpy by using a dedicated solver.
- Enforce the resulting temperature profile, but keep the species profiles to their initial values (Eqs. (3) and (4)).
It is found by using Cantera that the peak adiabatic temperature obtained at the fuel/air interface is , leading to the profiles shown in the initial fields of vorticity, temperature, mass fraction of , mass fraction of and the initial profiles of temperature and of mass fractions. Users are encouraged to implement the exact initial profiles from the benchmark website to facilitate later comparisons.
The resulting, initial configuration thus involves multiple species at different temperatures with steep profiles, just like in a real flame. However, for the present simulation, the reaction source terms are all still set to zero, as already mentioned: only convection and diffusion processes are considered. In order to enable benchmark computations also with DNS codes that do not provide advanced diffusion models, only constant values of the Lewis numbers are considered; for the same reason, thermodiffusion (Soret effect) is neglected on purpose (though it would be obviously relevant for such cases involving hydrogen as a fuel). The authors are fully aware that this is a crude approximation of reality[2]. But it must be kept in mind that the focus is set here on the detailed comparison between different codes and algorithms, and not on the resulting flow or flame structure. Hence, in this and in the next section, the values of the Lewis number for each species given in the table below shall be used to enable direct comparisons. These values have been estimated from a separate DNS for a similar configuration using the mixture-averaged diffusion model. The precise description of the associated thermodynamic state is available on the website.
| Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lewis number | 0.3290 | 0.2228 | 1.2703 | 0.8279 | 0.8128 | 1.0741 | 1.2582 | 1.2665 | 1.8268 |
On the other hand, considering that implementing advanced models for fluid viscosity and thermal conductivity is not difficult and does not increase the computational time significantly, local values of these quantities depending on composition and temperature should be taken into account; More specifically, and to ease further comparisons, the same models for viscosity and conductivity have been retained in the three participating codes, i.e. the mixture averaged formalism that is used in both the Cantera and Chemkin packages.
The viscosity is variable in the computational domain as well as with time and the Reynolds number of this configuration can only be estimated using the minimal value of viscosity (which is obtained in the air at 300 K, ), leading to which guarantees a laminar flow.
Concerning resolution, it is also suggested to keep a conservative resolution in time for this benchmark, corresponding to and . In space, each direction should be resolved by approximately 256 grid points, leading to a spatial resolution .
The reference time scale is here . The simulation should be performed for a physical time of at least as most of the results presented in this article are taken from this instant.
Initial fields of vorticity magnitude, temperature, mass fractions of , and mass fraction of (from left to right), for the 3-D, non-reacting case (Step 3).
Initial profiles of temperature and of mass fractions at and for the 3-D, non-reacting case (Step 3).
Kinetics
The kinetic scheme of Boivin et al.[1] which contains 9 species and 12 reactions has been used for this Benchmark.
This mechanism is provided here in the Cantera format:
If you are using Chemkin, please use the following files:
Transport and thermodynamic properties used for this Step
\cf{I think we all used the same transport (viscosity) and thermodynamic properties, but we should check again and compare that the implementations used in cantera/Chemkin provide the same values.}
\gl{Good idea: we could use 3 or 4 relevant reference compositions to quantify the discrepancies between Cantera and Chemkin (on rho, Cp, conductivity, viscosity for example) and also put these on the website.}
Put the transport data here
Aside suggestions
TBD
References
- ↑ 1.0 1.1 1.2
P. Boivin, C. Jiménez, A.L. Sanchez,, F.A. Williams, An explicit reduced mechanism for -air combustion., Proc. Combust. Inst. 33:517--523, 2011, BibtexAuthor : P. Boivin, C. Jiménez, A.L. Sanchez,, F.A. Williams
Title : An explicit reduced mechanism for -air combustion.
In : Proc. Combust. Inst. -
Address :
Date : 2011 - ↑
R. Hilbert, F. Tap, H. El-Rabii,, D. Thévenin, Impact of detailed chemistry and transport models on turbulent combustion simulations, Prog. Energ. Combust. Sci. 30:61--117, 2004, BibtexAuthor : R. Hilbert, F. Tap, H. El-Rabii,, D. Thévenin
Title : Impact of detailed chemistry and transport models on turbulent combustion simulations
In : Prog. Energ. Combust. Sci. -
Address :
Date : 2004